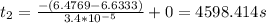At 25∘c, the decomposition of dinitrogen pentoxide, n2o5(g), into no2(g) and o2(g) follows first-order kinetics with k=3.4×10−5 s−1. a sample of n2o5 with an initial pressure of 760 torr decomposes at 25∘c until its partial pressure is 650 torr. how much time (in seconds) has elapsed? at 25, the decomposition of dinitrogen pentoxide, (g), into (g) and (g) follows first-order kinetics with . a sample of with an initial pressure of 760 decomposes at 25 until its partial pressure is 650 . how much time (in seconds) has elapsed? 5.3×10−6 2000 4600 34,000 190,000

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
At 25∘c, the decomposition of dinitrogen pentoxide, n2o5(g), into no2(g) and o2(g) follows first-ord...
Questions






Chemistry, 21.11.2019 05:31



Health, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31



English, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31



Social Studies, 21.11.2019 05:31

Social Studies, 21.11.2019 05:31

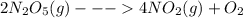
![-\frac{d[B]}{dt}=k[B] - - - -\frac{d[B]}{[B]}=k*dt](/tpl/images/0320/7058/5f6ff.png)
![-\frac{d[P(B)]}{P(B)}=k*dt](/tpl/images/0320/7058/d243b.png)
![-\frac{d[P(N_{2}O_{5})]}{P(N_{2}O_{5})}=k*dt](/tpl/images/0320/7058/282a3.png)
![\int\limits^p \,-\frac{d[P(N_{2}O_{5})]}{P(N_{2}O_{5})}=\int\limits^ t k*dt](/tpl/images/0320/7058/1b0c7.png)
![-(ln[P(N_{2}O_{5})]-ln[P(N_{2}O_{5})_{o})])=k(t_{2}-t_{1})](/tpl/images/0320/7058/3450d.png)
![\frac{-(ln[P(N_{2}O_{5})]-ln[P(N_{2}O_{5})_{o})])}{k}+t_{1}=t_{2}](/tpl/images/0320/7058/b0e0f.png)
![ln[P(N_{2}O_{5})]=ln(650)=6.4769](/tpl/images/0320/7058/0a747.png)
![ln[P(N_{2}O_{5})_{o}]=ln(760)=6.6333](/tpl/images/0320/7058/11e7e.png)