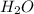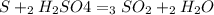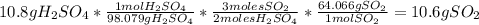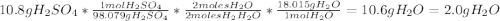Chemistry, 15.10.2019 04:00 johnnyboy41706
For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of 10.8 g of the second reactant. (a) s(s) + h2so4(aq) → so2(g) + h2o(l) so2 webassign will check your answer for the correct number of significant figures. g h2o webassign will check your answer for the correct number of significant figures. g

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2


Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

You know the right answer?
For each of the following unbalanced equations, calculate how many grams of each product would be pr...
Questions

Mathematics, 14.07.2021 17:50

Social Studies, 14.07.2021 17:50

Biology, 14.07.2021 17:50


Mathematics, 14.07.2021 17:50


Physics, 14.07.2021 17:50





Mathematics, 14.07.2021 17:50

History, 14.07.2021 17:50



English, 14.07.2021 18:00


English, 14.07.2021 18:00

Mathematics, 14.07.2021 18:00

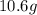 of
of 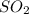
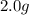 of
of