
Chemistry, 15.10.2019 04:30 smmailloux2335
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation when the concentration is doubled 1) the reaction rate is double with respect to that reactant 2) the order doesnt effect the reaction so there will be no change 3) the reaction rate quadruples with respect to that reactant. 4) the reaction rate isn't changed by individual reactants so there will be no change.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation wh...
Questions


Geography, 05.05.2020 17:22

Mathematics, 05.05.2020 17:22




English, 05.05.2020 17:22





Medicine, 05.05.2020 17:22

Chemistry, 05.05.2020 17:22



Mathematics, 05.05.2020 17:22




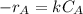
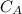 power is one (order 1), this could be proved just by checking it out in the rate law.
power is one (order 1), this could be proved just by checking it out in the rate law.


