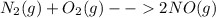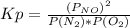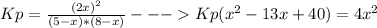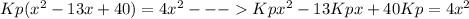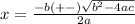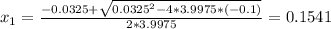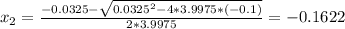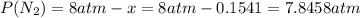Chemistry, 15.10.2019 05:30 elizabethburkha
Consider the reaction n2(g) + o2(g) â 2 no(g) k = 0.0025 a rigid container initially contains 8.00 atm of nitrogen and 5.00 atm of oxygen. what will be the partial pressure of nitrogen at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Consider the reaction n2(g) + o2(g) â 2 no(g) k = 0.0025 a rigid container initially contains 8.00 a...
Questions


SAT, 30.11.2021 02:30


Biology, 30.11.2021 02:30

Mathematics, 30.11.2021 02:30






English, 30.11.2021 02:30


Social Studies, 30.11.2021 02:30






Mathematics, 30.11.2021 02:30