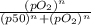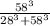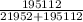Chemistry, 15.10.2019 05:30 skybrinson04
Calculate the fractional saturation for hemoglobin when the partial pressure of oxygen is 58 mm hg. assume hemoglobin is 50% saturated with oxygen at a partial pressure of 28 mm hg and that the hill coefficient is 3. express your answer to two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
Calculate the fractional saturation for hemoglobin when the partial pressure of oxygen is 58 mm hg....
Questions

Mathematics, 03.06.2020 20:04

Mathematics, 03.06.2020 20:04

Social Studies, 03.06.2020 20:04




Mathematics, 03.06.2020 20:05

Arts, 03.06.2020 20:05

Mathematics, 03.06.2020 20:05

Mathematics, 03.06.2020 20:05


Social Studies, 03.06.2020 20:05


Mathematics, 03.06.2020 20:05


Mathematics, 03.06.2020 20:05

Mathematics, 03.06.2020 20:05

History, 03.06.2020 20:05


Mathematics, 03.06.2020 20:05