
Chemistry, 15.10.2019 17:20 joelpimentel
In 1897 the swedish explorer andree tried to reach the north pole in a balloon. the balloon was filled with hydrogen gas. the hydrogen gas was prepared from iron splints and diluted sulfuric acid. the reaction is given below. fe(s) + h2so4(aq) feso4(aq) + h2(g)the volume of the balloon was 4870 m3 and the loss of hydrogen gas during filling was estimated at 20.%. what mass of iron splints and 95% (by mass) h2so4 were needed to ensure the complete filling of the balloon? assume a temperature of 0°c, a pressure of 1.0 atm during filling, and 100% yield. iron splints = % sulfuric acid =

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
In 1897 the swedish explorer andree tried to reach the north pole in a balloon. the balloon was fill...
Questions

Physics, 01.01.2020 06:31

English, 01.01.2020 06:31

Mathematics, 01.01.2020 06:31


Mathematics, 01.01.2020 06:31


History, 01.01.2020 06:31

History, 01.01.2020 06:31





Mathematics, 01.01.2020 06:31







Biology, 01.01.2020 06:31

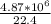 = 2,17 *10^5 moles (if 100% of the H2 is used)
= 2,17 *10^5 moles (if 100% of the H2 is used)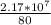 = 2,68*10^5 moles
= 2,68*10^5 moles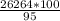 = 27646.32 kg
= 27646.32 kg

