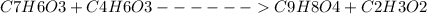Chemistry, 15.10.2019 18:00 kierafisher05
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol, and acetic anhydride ( c 4 h 6 o 3 ), which has a molar mass of 102.04 g/mol. the density of acetic anhydride is 1.082 g/ml. c 7 h 6 o 3 + c 4 h 6 o 3 ⟶ c 9 h 8 o 4 + c 2 h 3 o 2 what is the yield of aspirin ( c 9 h 8 o 4 ), which has a molar mass of 180.15 g/mol, possible when reacting 2.18 g of salicylic acid with 1.01 ml of acetic anhydride?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1


You know the right answer?
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol,...
Questions

Mathematics, 16.11.2020 08:20

Physics, 16.11.2020 08:20



Arts, 16.11.2020 08:20




Biology, 16.11.2020 08:20




Mathematics, 16.11.2020 08:20


Chemistry, 16.11.2020 08:20




Arts, 16.11.2020 08:20

English, 16.11.2020 08:20