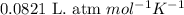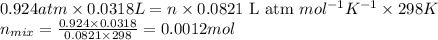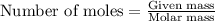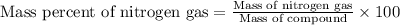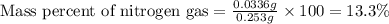Chemistry, 15.10.2019 17:30 cowerdwhisper23
The product gas is then passed through a concentrated solution of koh to remove the co2. after passage through the koh solution, the gas contains n2 and is saturated with water vapor. in a given experiment a 0.253-g sample of a compound produced 31.8 ml n2 saturated with water vapor at 258c and 726 torr. what is the mass percent of nitrogen in the compound

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1


Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
The product gas is then passed through a concentrated solution of koh to remove the co2. after passa...
Questions

Biology, 16.12.2020 17:50

Biology, 16.12.2020 17:50

Spanish, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50




Mathematics, 16.12.2020 17:50


Mathematics, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50

Mathematics, 16.12.2020 17:50





![\text{Compound}\xrightarrow[CuO(s)]{Hot}N_2(g)+CO_2(g)+H_2O(g)](/tpl/images/0322/3140/510d7.png)
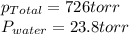
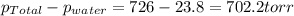
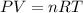
![25^oC=[25+273]K=298K](/tpl/images/0322/3140/df1f6.png)