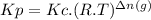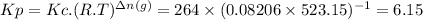Chemistry, 15.10.2019 18:20 thestarlexyp32wpj
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the other phosphorus compounds, coexist in equilibrium through the reactionpcl3(g) + cl2(g) = pcl5(g)at 250 ᵒc , an equilibrium mixture in a 25.0 l flask contains 0.105 g pcl5, 0.220 g pcl3 and 2.12 g of cl2. what are the values of(a) kc(b) kp for this reaction at 250 ᵒc ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1


Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the oth...
Questions


Social Studies, 26.07.2019 11:30


History, 26.07.2019 11:30




History, 26.07.2019 11:30

Mathematics, 26.07.2019 11:30


Mathematics, 26.07.2019 11:30


Chemistry, 26.07.2019 11:30

Mathematics, 26.07.2019 11:30


Biology, 26.07.2019 11:30

Biology, 26.07.2019 11:30



History, 26.07.2019 11:30

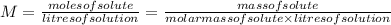
![[PCl_{3}]=\frac{0.220g}{137.5g/mol \times 25.0L } =6.40 \times 10^{-5} M](/tpl/images/0322/4252/44575.png)
![[Cl_{2}]=\frac{2.12g}{71.0g/mol \times 25.0L } =1.19 \times 10^{-3}M](/tpl/images/0322/4252/f9cf8.png)
![[PCl_{5}]=\frac{0.105g}{208.5g/mol \times 25.0L } =2.01 \times 10^{-5} M](/tpl/images/0322/4252/137a9.png)
![Kc=\frac{[PCl_{5}]}{[PCl_{3}]\times [Cl_{2}] } =\frac{2.01 \times 10^{-5} }{6.40 \times 10^{-5} \times 1.19 \times 10^{-3} } =264](/tpl/images/0322/4252/1590c.png)