
Chemistry, 15.10.2019 20:00 tejalawatson91
When a chemical reaction is at equilibrium, which of the following statements is always true?
a. the equilibrium constant keq= 1.
b. the ratio of the concentrations of reactants and products equals 1.
c. the rate of the forward reaction is equal to the rate of the backward reaction.
d. all of the choices are correct.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
When a chemical reaction is at equilibrium, which of the following statements is always true?
Questions


Biology, 14.11.2020 23:20


Advanced Placement (AP), 14.11.2020 23:20

Mathematics, 14.11.2020 23:20


Arts, 14.11.2020 23:20

Mathematics, 14.11.2020 23:20

Mathematics, 14.11.2020 23:20

Physics, 14.11.2020 23:20

Chemistry, 14.11.2020 23:20

Health, 14.11.2020 23:20

Biology, 14.11.2020 23:20

Computers and Technology, 14.11.2020 23:30


Social Studies, 14.11.2020 23:30


Mathematics, 14.11.2020 23:30

Mathematics, 14.11.2020 23:30

Mathematics, 14.11.2020 23:30

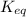
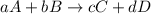
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0322/7004/9c8b0.png)
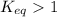 ; the reaction is product favored.When
; the reaction is product favored.When 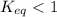 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 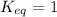 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

