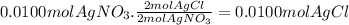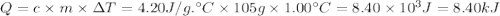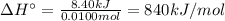Chemistry, 15.10.2019 20:30 coryintheswamp
10. when 50.0 ml of 0.200 m agno3 and 50.0 ml of 0.100 m cacl2, both at 25.0°c, are reacted in a coffee-cup calorimeter, the temperature of the reacting mixture increases to 26.0°c. calculate ∆h in kj per mole of agcl produced. assume the density of the solution is 1.05 g/ml and the specific heat capacity of the solution 4.20 j/g°c. agno3(aq) + hcl(aq) à agcl(s) + hno3(aq)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
10. when 50.0 ml of 0.200 m agno3 and 50.0 ml of 0.100 m cacl2, both at 25.0°c, are reacted in a cof...
Questions

Arts, 05.11.2020 14:00


Mathematics, 05.11.2020 14:00


Social Studies, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00




Health, 05.11.2020 14:00

Physics, 05.11.2020 14:00

English, 05.11.2020 14:00


Biology, 05.11.2020 14:00

Computers and Technology, 05.11.2020 14:00


Mathematics, 05.11.2020 14:00