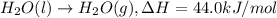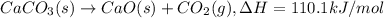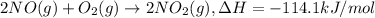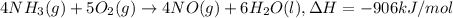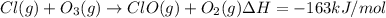Chemistry, 15.10.2019 22:00 Ashley606hernandez
Select whether there is no work done by the system, work done by the system or work done by the surroundings for each system described below:
a. h2o (l) ⟶ h2o (g) δh = 44.0 kj/mol b. 2 no (g) + o2 (g) ⟶ 2 no2(g) δh = -114.1 kj/mol c. cl (g) + o3 (g) ⟶ clo(g) + o2(g) δh = -163 kj/mol d. caco3 (s) ⟶ cao (s) + co2(g) δh = 110.1 kj/mol e. 4 nh3(g) + 5 o2(g) ⟶ 4 no (g) + 6 h2o(l) δh = -906 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Select whether there is no work done by the system, work done by the system or work done by the surr...
Questions

Mathematics, 12.03.2021 04:40

Chemistry, 12.03.2021 04:40

Mathematics, 12.03.2021 04:40

English, 12.03.2021 04:40



History, 12.03.2021 04:40



History, 12.03.2021 04:40

Computers and Technology, 12.03.2021 04:40

Mathematics, 12.03.2021 04:40


Mathematics, 12.03.2021 04:40

Mathematics, 12.03.2021 04:40



Social Studies, 12.03.2021 04:40

Mathematics, 12.03.2021 04:40

Social Studies, 12.03.2021 04:40