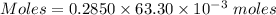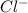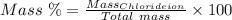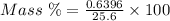Chemistry, 16.10.2019 00:00 Greghairston4839
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with agno₃ solution, causing a precipitation reaction. an indicator is used to detect the end point, which occurs when free ag⁺ ion is present in solution after all the cl⁻ has reacted. if 63.30 ml of 0.2850 m agno₃ is required to reach the end point, what is the mass percent of cl⁻ in the seawater (d of seawater = 1.024 g/ml)?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1
You know the right answer?
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with ag...
Questions


Mathematics, 20.05.2021 06:20

Mathematics, 20.05.2021 06:20


Social Studies, 20.05.2021 06:20

Mathematics, 20.05.2021 06:20



Biology, 20.05.2021 06:20

Mathematics, 20.05.2021 06:20





Biology, 20.05.2021 06:20

Biology, 20.05.2021 06:20


Mathematics, 20.05.2021 06:20

English, 20.05.2021 06:20

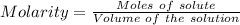
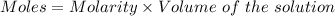
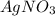 :
: