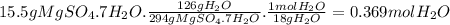Chemistry, 16.10.2019 01:30 Serenitybella
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrate). when heated, water is released as vapor and anhydrous mgso 4 remains. determine how many moles of vapor are released when 15.5 g of mgso 4 ·7h 2 o are heated. hint: include h 2 o when calculating the molar mass.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrat...
Questions




Mathematics, 08.12.2020 05:10




History, 08.12.2020 05:10

Mathematics, 08.12.2020 05:10

Mathematics, 08.12.2020 05:10



Advanced Placement (AP), 08.12.2020 05:10

Mathematics, 08.12.2020 05:10

Mathematics, 08.12.2020 05:10


Social Studies, 08.12.2020 05:10

SAT, 08.12.2020 05:10

Medicine, 08.12.2020 05:10

Mathematics, 08.12.2020 05:10