Chemistry, 16.10.2019 04:00 Fatimaneedhelp
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gibbs energy is -5798 kj/mol. assume ∆h does not change to estimate the additional non-expansion work that may be obtained by raising the temperature to blood temperature, 37o c. enter your answer in kj/mol to two significant figures and do not enter the units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gi...
Questions






English, 04.04.2020 11:10




Mathematics, 04.04.2020 11:11


Health, 04.04.2020 11:11








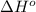 = -5645 kJ/mol
= -5645 kJ/mol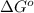 = -5798 kJ/mol
= -5798 kJ/mol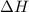 and
and 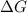 are as follows.
are as follows.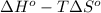
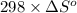
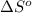 = 0.513 kJ/mol K
= 0.513 kJ/mol K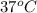 = (37 + 273) K = 310 K
= (37 + 273) K = 310 K