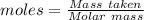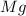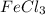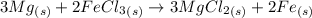Chemistry, 16.10.2019 06:00 jessica28757
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3mg(s) + 2fecl3(s) → 3mgcl2(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. what mass of magnesium chloride = 95.21 g/mol) is formed?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
You know the right answer?
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofi...
Questions



Chemistry, 20.10.2020 21:01


Chemistry, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01


English, 20.10.2020 21:01

Social Studies, 20.10.2020 21:01




Advanced Placement (AP), 20.10.2020 21:01


Biology, 20.10.2020 21:01

English, 20.10.2020 21:01