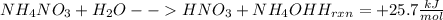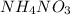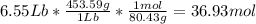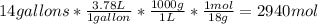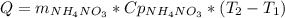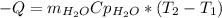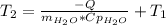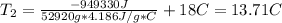Acertain commercial process relies on the addition of nh4no3 to a solution of their proprietary compound. the junior apprentice chemist develops a plan to add 6.55 pounds of nh4no3 to 14.0 gallons of water. the water in the factory is typically at an initial temperature of 18.0 c, and the temperature of the water cannot drop below 15 c, or the reaction will be too slow. will the above plan work? use the value of delta hrxn = +25.7 kj/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
Acertain commercial process relies on the addition of nh4no3 to a solution of their proprietary comp...
Questions

History, 19.01.2021 21:50









Law, 19.01.2021 21:50

Mathematics, 19.01.2021 21:50

Mathematics, 19.01.2021 21:50





History, 19.01.2021 21:50

Mathematics, 19.01.2021 21:50