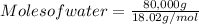Submit your answer for the remaining reagent in tutorial assignment #1 question 9 here, including units. note: use e for scientific notation formatting (ie, write 1.23e4 for 1.23x10^4). 9. b. identify the mass of excess reagent remaining in the following system: lithium oxide is used aboard the space shuttle to remove water from the air supply according to the equation: li2o(s) + h2o(0) ► 2lioh(s). in this system, 80.0kg of water is to be removed and 65.0kg of li2o is available.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 06:30
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent.true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight.a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

You know the right answer?
Submit your answer for the remaining reagent in tutorial assignment #1 question 9 here, including un...
Questions

Mathematics, 12.11.2020 21:30



Mathematics, 12.11.2020 21:30

Geography, 12.11.2020 21:30


Spanish, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30


History, 12.11.2020 21:30


History, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30





Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30