Calculate the solubility in mol/l of pbbr2(s) (ksp = 2.1 • 10-6, mw = 367.0 g/mol)
(b) c...

Chemistry, 16.10.2019 21:10 burnsmykala23
Calculate the solubility in mol/l of pbbr2(s) (ksp = 2.1 • 10-6, mw = 367.0 g/mol)
(b) convert your answer to part a to g/100 ml
(c) what is the concentration (in mol/l) of pbbr2(s) given that its density is 6.66 g/ml?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
Questions

English, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01

Chemistry, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Biology, 22.10.2020 22:01

English, 22.10.2020 22:01


Chemistry, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01






Social Studies, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01

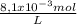 ×
×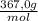 ×
×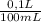 = 0,297 g/100mL
= 0,297 g/100mL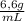 ×
×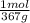 ×
×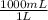 = 18,0 mol/L
= 18,0 mol/L

