
Chemistry, 16.10.2019 21:20 sdwhitneyhillis
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one reactant a is given by: = k [aj? -d[a/dt where k is the reaction rate coefficient. derive an expression for the half-life of a in terms of k and the concentration of a at time t-0 (ao)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one rea...
Questions


Computers and Technology, 09.03.2021 05:10







Physics, 09.03.2021 05:10


History, 09.03.2021 05:10



English, 09.03.2021 05:10

Mathematics, 09.03.2021 05:10

Mathematics, 09.03.2021 05:10

History, 09.03.2021 05:10



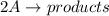
![\textrm{rate}=-\dfrac{\Delta[\textrm A]}{2\Delta t}=k[\textrm A]^2](/tpl/images/0326/2107/66a24.png)
![\dfrac{d[A]}{dt}=-k[A]^2](/tpl/images/0326/2107/6f64e.png)
![\int_{[A_t]}^{[A_0]}\frac{d[A]}{[A]^2}=-\int_{0}^{t}kdt](/tpl/images/0326/2107/7af1b.png)
![\dfrac{1}{[A]} = \dfrac{1}{[A]_0}+kt](/tpl/images/0326/2107/704d4.png)
![[A_t]](/tpl/images/0326/2107/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0326/2107/9a686.png) is the initial concentration
is the initial concentration![[A_t]=\frac{1}{2}\times [A_0]](/tpl/images/0326/2107/09fb2.png)
![t_{1/2}=\dfrac{1}{k[A_o]}](/tpl/images/0326/2107/4b6c5.png)



