
Chemistry, 16.10.2019 21:30 itzdulceee
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=−log[h+] due to the autoionization of water, in any aqueous solution, the hydrogen ion concentration and the hydroxide ion concentration, [oh−], are related to each other by the kw of water: kw=[h+][oh−]=1.00×10−14 where 1.00×10−14 is the value at approximately 297 k. based on this relation, the ph and poh are also related to each other as 14.00=ph+poh the temperature for each solution is carried out at approximately 297 k where kw=1.00×10−14. part a 0.35 g of hydrogen chloride (hcl) is dissolved in water to make 2.5 l of solution. what is the ph of the resulting hydrochloric acid solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=...
Questions

Mathematics, 27.06.2019 01:00

Health, 27.06.2019 01:00








Mathematics, 27.06.2019 01:00


Biology, 27.06.2019 01:00


Arts, 27.06.2019 01:00

Mathematics, 27.06.2019 01:00




Biology, 27.06.2019 01:00

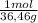 = 9,6x10⁻³ moles HCl ≡ moles H⁺
= 9,6x10⁻³ moles HCl ≡ moles H⁺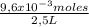 = 3,84x10⁻³M
= 3,84x10⁻³M

