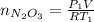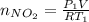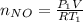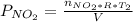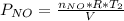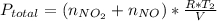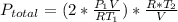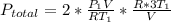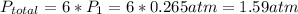Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is tripled, the gas completely decomposes, producing no2(g) and no(g). calculate the final pressure of the gas mixture, assuming that the container volume does not change.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is t...
Questions

Mathematics, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30

Business, 01.10.2019 09:30

Physics, 01.10.2019 09:30


History, 01.10.2019 09:30



English, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30

French, 01.10.2019 09:30


Mathematics, 01.10.2019 09:30


Mathematics, 01.10.2019 09:30


Physics, 01.10.2019 09:30

Chemistry, 01.10.2019 09:30


English, 01.10.2019 09:30

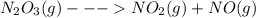
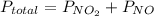
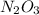 because it decomposed completely.
because it decomposed completely.