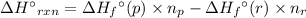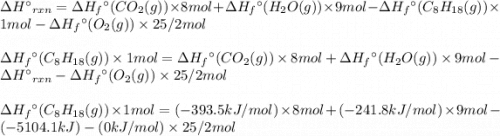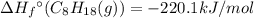Chemistry, 17.10.2019 03:20 mariahdelossantos031
For a particular isomer of c8h18, the combustion reaction produces 5104.1 kj of heat per mole of c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5104.1 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
For a particular isomer of c8h18, the combustion reaction produces 5104.1 kj of heat per mole of c8h...
Questions

Mathematics, 20.01.2021 19:00

Mathematics, 20.01.2021 19:00




English, 20.01.2021 19:00

Mathematics, 20.01.2021 19:00

Mathematics, 20.01.2021 19:00

History, 20.01.2021 19:00




Mathematics, 20.01.2021 19:00


Mathematics, 20.01.2021 19:00

Arts, 20.01.2021 19:00

History, 20.01.2021 19:00