
Chemistry, 17.10.2019 05:00 trinityanne1738
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600 m. n2(g)+o2(g)↽−−⇀2no(g) if more no is added, bringing its concentration to 0.900 m, what will the final concentration of no be after equilibrium is re‑established?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600...
Questions



Computers and Technology, 10.10.2019 01:00

















![K_{eq}=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}} \\K_{eq}=\frac{0.6^2}{0.2*0.2}\\ K_{eq}=9](/tpl/images/0327/3782/fd314.png)
![9=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}}\\9=\frac{[0.9+2x]^2}{[0.2-x][0.2-x]}](/tpl/images/0327/3782/c867b.png)
![\frac{1}{9} =\frac{[N_2]_{eq}[O_2]_{eq}}{[NO]^2_{eq}}\\\frac{1}{9} =\frac{[0.2+x][0.2+x]}{[0.9-2x]^2}](/tpl/images/0327/3782/3755f.png)
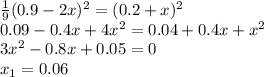
![[NO]_{eq}=0.9-0.06=0.84M](/tpl/images/0327/3782/19d1e.png)


