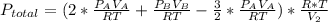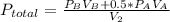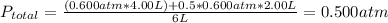Chemistry, 17.10.2019 05:20 janeekajones08
2. a 2.00 l flask containing 0.600 atm of gas a is connected to a 4.00 l flask containing 0.600 atm of gas b, after which the valve between the flasks is opened so that the gases can mix and react according to the following reaction equation. if the theoretical yield is produced at constant temperature, the total pressure in the combined flasks should 2a(g) + 3b(g) → a2b3(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
2. a 2.00 l flask containing 0.600 atm of gas a is connected to a 4.00 l flask containing 0.600 atm...
Questions

Mathematics, 16.10.2020 06:01





Computers and Technology, 16.10.2020 06:01


Computers and Technology, 16.10.2020 06:01





Biology, 16.10.2020 06:01


Business, 16.10.2020 06:01

History, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01


Mathematics, 16.10.2020 06:01

Biology, 16.10.2020 06:01

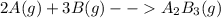
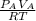
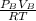
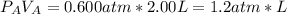

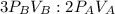
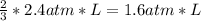 of A
of A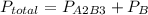
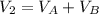
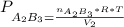
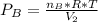
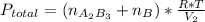
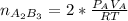
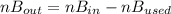
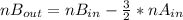
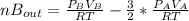
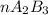 and nB in the total pressure equation we get:
and nB in the total pressure equation we get: