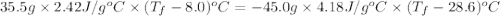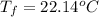Chemistry, 17.10.2019 06:00 steven2996
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (density = 1.0 g/ml) initially at 28.6 ∘c in an insulated beaker, what is the final temperature of the mixture, assuming that no heat is lost? (cetoh=2.42j/(g⋅∘

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

You know the right answer?
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (den...
Questions



Chemistry, 09.02.2021 22:00

Biology, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00




Mathematics, 09.02.2021 22:00



Biology, 09.02.2021 22:00

Biology, 09.02.2021 22:00

French, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00

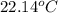
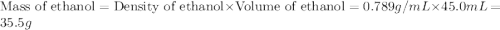
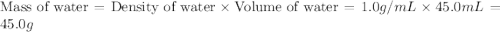
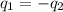
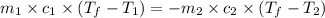
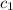 = specific heat of ethanol =
= specific heat of ethanol = 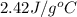
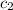 = specific heat of water =
= specific heat of water = 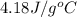
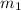 = mass of ethanol = 35.5 g
= mass of ethanol = 35.5 g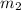 = mass of water = 45.0 g
= mass of water = 45.0 g = final temperature of mixture = ?
= final temperature of mixture = ?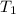 = initial temperature of ethanol =
= initial temperature of ethanol = 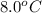
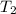 = initial temperature of water =
= initial temperature of water =