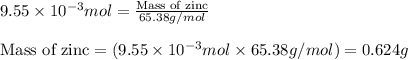Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydrochloric acid to metallic zinc. typically, the hydrogen gas is bubbled through water for collection and becomes saturated with water vapor. suppose 240. ml of hydrogen gas is collected at 30. c and has a total pressure of 1.032 atm by this process. what is the partial pressure of hydrogen gas in the sample? how many grams of zinc must have reacted to produce this quantity of hydrogen? (the vapor pressure of water is 32 torr at 30 c).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2
You know the right answer?
Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydroch...
Questions



History, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

Physics, 07.10.2019 22:30

Computers and Technology, 07.10.2019 22:30



English, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30


English, 07.10.2019 22:30


Chemistry, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30



Mathematics, 07.10.2019 22:30

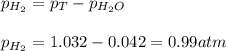
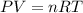
![30^oC=[30+273]K=303K](/tpl/images/0328/9260/fd4b3.png)
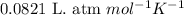
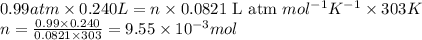
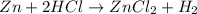
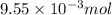 of hydrogen gas is produced from =
of hydrogen gas is produced from = 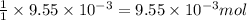 of zinc metal
of zinc metal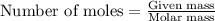
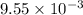 moles
moles