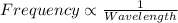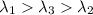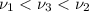One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of 2.12 10-10 m, and another type of electromagnetic radiation has photons with energy equal to 3.97 10-19 j/photon. identify each type of electromagnetic radiation. 107.1 mhz 2.12 10-10 m 3.97 10-19 j/photon fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light rank them in order of increasing photon energy and increasing frequency

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of...
Questions

Computers and Technology, 01.11.2020 22:20

Chemistry, 01.11.2020 22:20

Mathematics, 01.11.2020 22:20


Physics, 01.11.2020 22:20

Business, 01.11.2020 22:20

Physics, 01.11.2020 22:20




Mathematics, 01.11.2020 22:20

Mathematics, 01.11.2020 22:20


Mathematics, 01.11.2020 22:20



Biology, 01.11.2020 22:20


SAT, 01.11.2020 22:20

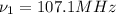
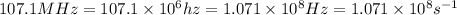
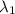
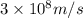
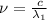
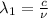
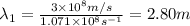
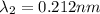 (X-rays)
(X-rays)
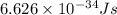
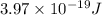
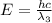
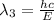
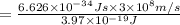
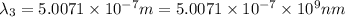
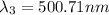 (visible light)
(visible light)