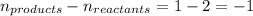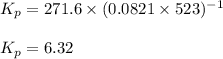Chemistry, 17.10.2019 18:00 pippalotta
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the other phosphorus compounds, coexist in equilibrium through the reaction
pcl3(g) + cl2(g) = pcl5(g)
at 250 ᵒc , an equilibrium mixture in a 25.0 l flask contains 0.105 g pcl5, 0.220 g pcl3 and 2.12 g of cl2. what are the values of
a) kc
b) kp for this reaction at 250 ᵒc ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

You know the right answer?
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the oth...
Questions

Mathematics, 28.11.2019 00:31


Computers and Technology, 28.11.2019 00:31



Mathematics, 28.11.2019 00:31


Mathematics, 28.11.2019 00:31


Social Studies, 28.11.2019 00:31



Social Studies, 28.11.2019 00:31

Computers and Technology, 28.11.2019 00:31


English, 28.11.2019 00:31

Spanish, 28.11.2019 00:31



History, 28.11.2019 00:31

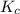 for the given reaction is 271.6
for the given reaction is 271.6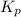 for the reaction is 6.32
for the reaction is 6.32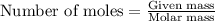 .....(1)
.....(1)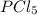 :
: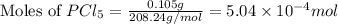
 :
: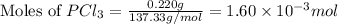
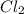 :
: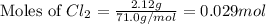
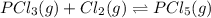
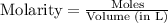
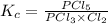
![[PCl_5]=\frac{5.04\times 10^{-4}mol}{25L}](/tpl/images/0328/9204/d1b89.png)
![[PCl_3]=\frac{1.60\times 10^{-3}mol}{25L}](/tpl/images/0328/9204/16be6.png)
![[Cl_2]=\frac{0.029mol}{25L}](/tpl/images/0328/9204/6d85a.png)
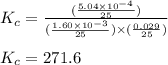
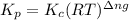
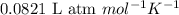
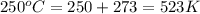
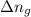 = change in number of moles of gas particles =
= change in number of moles of gas particles =