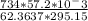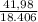At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. the oxygen gas collected over water occupied 57.2 ml at a temperature of 22ºc and a pressure of 734 torr. calculate the mass percent of nahco₃ in the original sample. (at 22ºc the vapor pressure of water is 19.8 torr.)

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0....
Questions

Social Studies, 10.11.2019 00:31

Mathematics, 10.11.2019 00:31

English, 10.11.2019 00:31

History, 10.11.2019 00:31

English, 10.11.2019 00:31

Mathematics, 10.11.2019 00:31

Mathematics, 10.11.2019 00:31


History, 10.11.2019 00:31


Geography, 10.11.2019 00:31


Mathematics, 10.11.2019 00:31


Mathematics, 10.11.2019 00:31

Health, 10.11.2019 00:31

Biology, 10.11.2019 00:31

Chemistry, 10.11.2019 00:31


Mathematics, 10.11.2019 00:31