Which one of the following statements is true?
a. the enthalpy change for a reaction is inde...

Chemistry, 17.10.2019 19:00 unknown235
Which one of the following statements is true?
a. the enthalpy change for a reaction is independent of the state of the reactants and products.
b. enthalpy is an intensive property.
c. enthalpy is a state function.
d. h is the value of q measured under conditions of constant volume.
e. the enthalpy change of a reaction is the reciprocal of the δh of the reverse reaction.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

You know the right answer?
Questions



Chemistry, 15.02.2022 14:00



Mathematics, 15.02.2022 14:00

Social Studies, 15.02.2022 14:00



Social Studies, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00




Physics, 15.02.2022 14:00


Biology, 15.02.2022 14:00


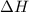 and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.
and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.

