
The decomposition reaction 2 hi(g) → h2(g) + 12(g), is second order and has a rate constant equal to 1.6 x 10m's at 700 °c. if the initial concentration of hi in the container is 3.4 x 10-m, how many minutes will it take for the concentration to be reduced to 8.0 x 104 m?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
The decomposition reaction 2 hi(g) → h2(g) + 12(g), is second order and has a rate constant equal to...
Questions




Computers and Technology, 18.07.2019 13:00


Mathematics, 18.07.2019 13:00




Mathematics, 18.07.2019 13:00


History, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00



History, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00


![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0329/9761/ccade.png)
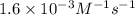
![[A_t]](/tpl/images/0329/9761/5262c.png) = final concentration =
= final concentration = 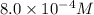
![[A_o]](/tpl/images/0329/9761/dc622.png) = initial concentration =
= initial concentration = 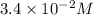
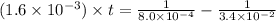
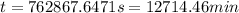 (1 min = 60 s)
(1 min = 60 s)

