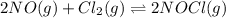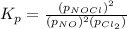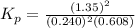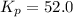Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2


Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
The partial pressures in an equilibrium mixture of no, cl2, and noci at 500 k are as follows: pno =...
Questions

Mathematics, 08.12.2020 01:30

Chemistry, 08.12.2020 01:30

Biology, 08.12.2020 01:30




Mathematics, 08.12.2020 01:30

Mathematics, 08.12.2020 01:30

History, 08.12.2020 01:30

Mathematics, 08.12.2020 01:30


Mathematics, 08.12.2020 01:30




Mathematics, 08.12.2020 01:30


Mathematics, 08.12.2020 01:30

History, 08.12.2020 01:30

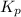 at temperature 500 K is 52.0
at temperature 500 K is 52.0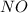 at equilibrium = 0.240 atm
at equilibrium = 0.240 atm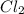 at equilibrium = 0.608 atm
at equilibrium = 0.608 atm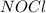 at equilibrium = 1.35 atm
at equilibrium = 1.35 atm