
Chemistry, 18.10.2019 01:00 19thomasar
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 500. k. (5.00 x 10k) calculate the value of kc at 500. k. for the same reaction, calculate the molar concentration of reactants and products at equilibrium if initially 1.00 mol of (ch3),cci was placed in a 5.00 l vessel.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 50...
Questions

Mathematics, 17.04.2021 07:50

Mathematics, 17.04.2021 07:50




Computers and Technology, 17.04.2021 07:50

Mathematics, 17.04.2021 08:00

Mathematics, 17.04.2021 08:00

History, 17.04.2021 08:00

Mathematics, 17.04.2021 08:00

Spanish, 17.04.2021 08:00


English, 17.04.2021 08:00

Mathematics, 17.04.2021 08:00

Mathematics, 17.04.2021 08:00

Social Studies, 17.04.2021 08:00



Mathematics, 17.04.2021 08:00

Mathematics, 17.04.2021 08:00

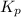 for the reaction is 6.32 and concentrations of
for the reaction is 6.32 and concentrations of 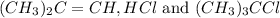 is 0.094 M, 0.094 M and 0.106 M respectively.
is 0.094 M, 0.094 M and 0.106 M respectively.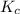 is given by the formula:
is given by the formula: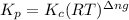
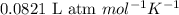
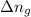 = change in number of moles of gas particles =
= change in number of moles of gas particles = 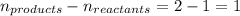
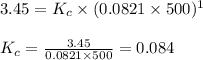
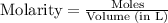
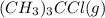 = 1.00 mol
= 1.00 mol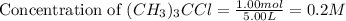
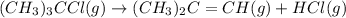
![K_c=\frac{[(CH_3)_2C=CH]\times [HCl]}{[(CH_3)_3CCl]}](/tpl/images/0329/9758/8a400.png)
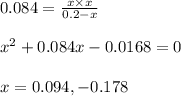
![[(CH_3)_2C=CH]=x=0.094M](/tpl/images/0329/9758/5587a.png)
![[HCl]=x=0.094M](/tpl/images/0329/9758/e3b5a.png)
![[(CH_3)_3CCl]=(0.2-x)=(0.2-0.094)=0.106M](/tpl/images/0329/9758/364df.png)


