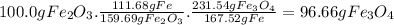calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol...

Chemistry, 18.10.2019 01:00 CameronVand21
calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol molar mass of fe3o4 = 231.54 g/mol hint: you need two conversion factors

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

You know the right answer?
Questions

Mathematics, 29.01.2021 23:30


Mathematics, 29.01.2021 23:30


Mathematics, 29.01.2021 23:30

Chemistry, 29.01.2021 23:30

History, 29.01.2021 23:30


Physics, 29.01.2021 23:30



Biology, 29.01.2021 23:30

Arts, 29.01.2021 23:30


Mathematics, 29.01.2021 23:30




Mathematics, 29.01.2021 23:30

Mathematics, 29.01.2021 23:30