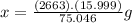Chemistry, 18.10.2019 00:30 jettskii214
For 2,663 kg of a compound with the formula al(so), determine the following quantities (4 pts each); a) the number of moles of the compound in this mass. b) the number of moles of sulfur atoms in this mass. c) the number of aluminum atoms in this mass. d) the mass of oxygen atoms in this mass.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
For 2,663 kg of a compound with the formula al(so), determine the following quantities (4 pts each);...
Questions

Mathematics, 26.08.2021 01:40

Mathematics, 26.08.2021 01:40

Mathematics, 26.08.2021 01:40



History, 26.08.2021 01:40

English, 26.08.2021 01:40







Computers and Technology, 26.08.2021 01:40

Social Studies, 26.08.2021 01:40

Mathematics, 26.08.2021 01:40

Social Studies, 26.08.2021 01:40

Mathematics, 26.08.2021 01:40

Mathematics, 26.08.2021 01:40

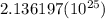 Al atoms
Al atoms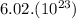 elemental units
elemental units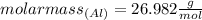
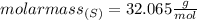
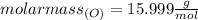
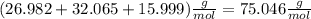
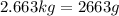
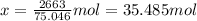
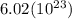
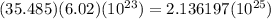 Al atoms
Al atoms