
Chemistry, 18.10.2019 01:10 jellybellyje
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more soluble in kerosene because the non polar structure of kerosene allows the smaller lons to infiltrate the structure of the larger compound and therefore dissolve. nacl will be more soluble in kerosene because kerosene can dissolve all compounds. nacl will be equally soluble in both solvent. nal will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more...
Questions



History, 13.05.2021 06:00



Biology, 13.05.2021 06:00


Mathematics, 13.05.2021 06:00


Chemistry, 13.05.2021 06:00

Mathematics, 13.05.2021 06:00

Mathematics, 13.05.2021 06:00

Mathematics, 13.05.2021 06:00





Mathematics, 13.05.2021 06:00

Biology, 13.05.2021 06:00

Mathematics, 13.05.2021 06:00

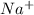 and
and 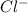 . As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
. As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.

