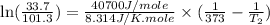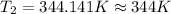Chemistry, 18.10.2019 01:20 dewayne5599
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure is 33.7 kpa. considering the normal boiling point of water (100 °c) and its heat of vaporization (40.7 kj/mol), at what temperature will water boil atop mount everest? m

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure...
Questions






Mathematics, 01.04.2020 13:15







Biology, 01.04.2020 13:18

Mathematics, 01.04.2020 13:19

Mathematics, 01.04.2020 13:19

Chemistry, 01.04.2020 13:19



English, 01.04.2020 13:36

Mathematics, 01.04.2020 13:36

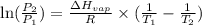
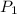 = atmospheric pressure at at sea level = 101.3 kPa
= atmospheric pressure at at sea level = 101.3 kPa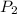 = atmospheric pressure at top mount everest = 33.7 kPa
= atmospheric pressure at top mount everest = 33.7 kPa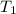 = normal boiling point of water =
= normal boiling point of water = 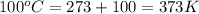
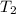 = temperature at top mount everest = ?
= temperature at top mount everest = ?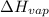 = heat of vaporization = 40.7 kJ/mole = 40700 J/mole
= heat of vaporization = 40.7 kJ/mole = 40700 J/mole