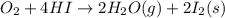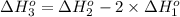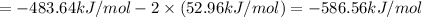Chemistry, 18.10.2019 03:30 lizchavarria863
9. calculate the standard enthalpy of the 3rd reaction using the given data: a, h° = +52.96 kj/mol afh= -483.64 kj/mol h2s)+i202 hi 2 h2g)+o2ig)2 h2o() 4 hig+o2lg)2 12)+2 h2o(e (1) (2) (3) a, h° =? 10. calculate the internal energy change for reaction (3) in the previous problem.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
9. calculate the standard enthalpy of the 3rd reaction using the given data: a, h° = +52.96 kj/mol...
Questions



Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00


Engineering, 10.06.2021 01:00


Mathematics, 10.06.2021 01:00

Social Studies, 10.06.2021 01:00


Mathematics, 10.06.2021 01:00


Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00


Mathematics, 10.06.2021 01:00

Engineering, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

 ...[1]
...[1]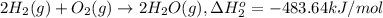 ...[2]
...[2]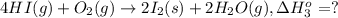 ..[3]
..[3]