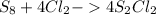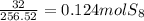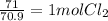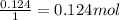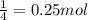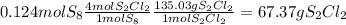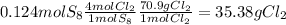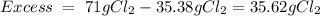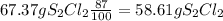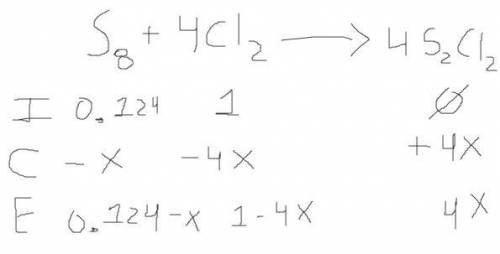Chemistry, 18.10.2019 03:30 ejfleck3655
6. write the ice chart for the reaction of 32.0 g of sulfur and 71.0 g of chlorine: s8 + 4 cl24s2cl2 after completing the chart give a. the mass of product b. the mass of agent in excess c. the mass produced if there is an 87.% yield

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

You know the right answer?
6. write the ice chart for the reaction of 32.0 g of sulfur and 71.0 g of chlorine: s8 + 4 cl24s2cl...
Questions

Mathematics, 28.01.2021 04:50

Mathematics, 28.01.2021 04:50

Biology, 28.01.2021 04:50


Mathematics, 28.01.2021 04:50

Mathematics, 28.01.2021 04:50

English, 28.01.2021 04:50


Mathematics, 28.01.2021 04:50

Mathematics, 28.01.2021 04:50


Mathematics, 28.01.2021 04:50

English, 28.01.2021 04:50



Mathematics, 28.01.2021 04:50

English, 28.01.2021 04:50

Mathematics, 28.01.2021 04:50

Mathematics, 28.01.2021 04:50

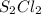
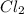
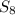 and the reactives are consumed, so for
and the reactives are consumed, so for