Chemistry, 18.10.2019 03:30 GamerGirl15
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxide gas and liquid water. a. write the balanced equation for the reaction b. if there are 0.500 moles of copper metal present how many moles of nitric acid are required for the reaction? how many moles of nitrogen dioxide gas are formed?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxid...
Questions




Social Studies, 22.08.2019 05:30

History, 22.08.2019 05:30



Computers and Technology, 22.08.2019 05:30


Chemistry, 22.08.2019 05:30

History, 22.08.2019 05:30


English, 22.08.2019 05:30

Mathematics, 22.08.2019 05:30

History, 22.08.2019 05:30





Biology, 22.08.2019 05:30

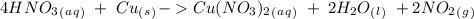
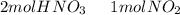
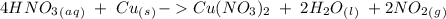
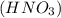 we have to use the molar ratio in the balence reaction:
we have to use the molar ratio in the balence reaction: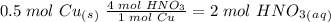
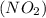 we have to follow the same logic:
we have to follow the same logic: