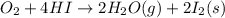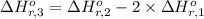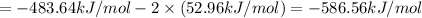Chemistry, 18.10.2019 03:30 miya257916
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº = +52.96 kj/mol 2 h2(g) + o2(g) + 2 h209) a, hº = - 483.64 kj/mol 4 hi) + o2(g) +2 12(8) + 2 h206) a, hº =? a. h=-589.5619/mol calculate the internal energy change for reaction (3) in the previous problem.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº...
Questions



Mathematics, 29.04.2021 17:40







Mathematics, 29.04.2021 17:40


Chemistry, 29.04.2021 17:40



Mathematics, 29.04.2021 17:40

Mathematics, 29.04.2021 17:40



Mathematics, 29.04.2021 17:40

 ...[1]
...[1]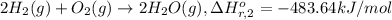 ...[2]
...[2]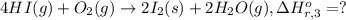 ..[3]
..[3]