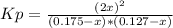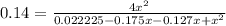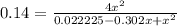Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) ⇌ 2no(g)the equilibrium constant kp for the reaction is 0.14 at 1200 °c. if a container is charged with 0.175 atm of nitrogen and 0.127 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of oxygen? report your answer to three significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions


Mathematics, 16.09.2019 10:10

History, 16.09.2019 10:10

Social Studies, 16.09.2019 10:10


History, 16.09.2019 10:10


English, 16.09.2019 10:10



Biology, 16.09.2019 10:10

History, 16.09.2019 10:10

Chemistry, 16.09.2019 10:10



History, 16.09.2019 10:10

Chemistry, 16.09.2019 10:10


Biology, 16.09.2019 10:10

Chemistry, 16.09.2019 10:10

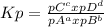 , where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table:
, where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table: