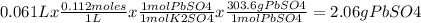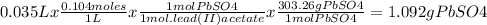Chemistry, 18.10.2019 19:00 chassidytjtrimb
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acetate solution and the following precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)? 2kc2h3o2(aq)+pbso4(s)
the solid pbso4 is collected, dried, and found to have a mass of 0.997g .
determine the limiting reactant, the theoretical yield, and the percent yield.
part a.
identify the limiting reactant.
kc2h3o2
pb(c2h3o2)2
k2so4
pbso4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

You know the right answer?
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acet...
Questions


History, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50


Biology, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50


Computers and Technology, 08.12.2020 05:50

Arts, 08.12.2020 05:50

English, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50


Biology, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50


Mathematics, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50