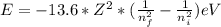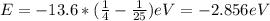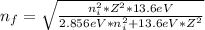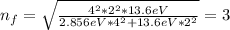Chemistry, 18.10.2019 18:30 gyexisromero10
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted. if an excited electron in an he+ ion falls from =4, which energy level must it fall to ) for blue light of a similar wavelength to be emitted?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted....
Questions




English, 12.10.2019 12:30


History, 12.10.2019 12:30






History, 12.10.2019 12:30





English, 12.10.2019 12:30

Mathematics, 12.10.2019 12:30

History, 12.10.2019 12:30