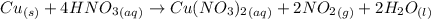Chemistry, 18.10.2019 19:10 densliverdensentos
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. what volume of nitrogen dioxide is formed at 724 torr and 28.2° c by reacting 4.84 cm3 of copper (d = 8.95 g/cm3) with 227 ml of nitric acid (d = 1.42 g/cm3, 68.0% hno3 by mass)?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Mathematics, 28.01.2020 19:09



Chemistry, 28.01.2020 19:09

Biology, 28.01.2020 19:09

Mathematics, 28.01.2020 19:09

Mathematics, 28.01.2020 19:09

Mathematics, 28.01.2020 19:09

Geography, 28.01.2020 19:09




English, 28.01.2020 19:09


History, 28.01.2020 19:09


History, 28.01.2020 19:09

Arts, 28.01.2020 19:09

Biology, 28.01.2020 19:09

Mathematics, 28.01.2020 19:09




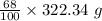 = 219.1912 g
= 219.1912 g