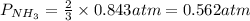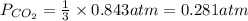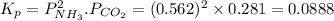Chemistry, 18.10.2019 19:10 daverius3153
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reaches equilibrium: nh2co2nh4(s) ⇀↽ 2 nh3(g) + co2(g) the total pressure in the closed container under these condition is found to be 0.843 atm. calculate a value for the equilibrium constant, kp. a) 0.00701 b) 0.0888 c) 0.222 d) 0.599

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

You know the right answer?
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reac...
Questions


History, 04.08.2019 15:00


English, 04.08.2019 15:00


Mathematics, 04.08.2019 15:00


Biology, 04.08.2019 15:00


English, 04.08.2019 15:00


Health, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00





History, 04.08.2019 15:00


Mathematics, 04.08.2019 15:00

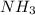 and
and 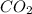 are gaseous. Hence equilibrium constant depends upon partial pressures of
are gaseous. Hence equilibrium constant depends upon partial pressures of 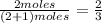
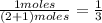
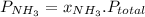 and P_{CO_{2}}= x_{CO_{2}}.P_{total}
and P_{CO_{2}}= x_{CO_{2}}.P_{total}