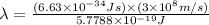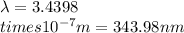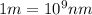Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because they carry enough energy to break bonds within the molecules. a typical carbon–carbon bond requires 348 kj/mol to break. what is the longest wavelength of radiation with enough energy to break carbon–carbon bonds?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

You know the right answer?
Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because t...
Questions


Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00



Mathematics, 04.06.2021 21:00


Chemistry, 04.06.2021 21:00





History, 04.06.2021 21:00



Chemistry, 04.06.2021 21:00


Chemistry, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00

History, 04.06.2021 21:00

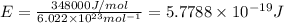
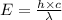
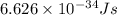
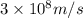
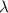 = wavelength of the radiation
= wavelength of the radiation