Chemistry, 18.10.2019 19:20 arwasoliman363
The partial pressure of ch4 is 0.175 atm and that of o2 is 0.250 atm in a mixture of the two gases. what is the mole fraction of each gas in the mixture? if the mixture occupies a volume of 10.5 l at 35oc, calculate the total number of moles of gas in the mixture. calculate the number of grams of each gas in the mixture.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
The partial pressure of ch4 is 0.175 atm and that of o2 is 0.250 atm in a mixture of the two gases....
Questions

Mathematics, 21.09.2019 21:00



Mathematics, 21.09.2019 21:00

Biology, 21.09.2019 21:00

English, 21.09.2019 21:00





Mathematics, 21.09.2019 21:00


Mathematics, 21.09.2019 21:00

Mathematics, 21.09.2019 21:00

Biology, 21.09.2019 21:00


Physics, 21.09.2019 21:00


Biology, 21.09.2019 21:00

Mathematics, 21.09.2019 21:00








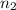


 (Dalton's law)
(Dalton's law)


 (Dalton's law)
(Dalton's law)