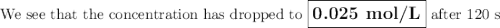Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
The decomposition of n2o5(g) —> no2(g) + no3(g) proceeds as a first order reaction with a half l...
Questions

History, 19.12.2019 08:31

History, 19.12.2019 08:31



Mathematics, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31

History, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Chemistry, 19.12.2019 08:31

English, 19.12.2019 08:31


Social Studies, 19.12.2019 08:31



History, 19.12.2019 08:31



Social Studies, 19.12.2019 08:31

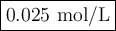
![\begin{array}{crcc}\textbf{No. of} && \textbf{Fraction} & \\\textbf{half-lives} & \textbf{t/s} & \textbf{remaining} &\rm \mathbf{{[N_{2}O_{5}] /(mol/L)}}\\0 & 0 & 1 & 0.400\\1 & 30.0 & 1/2 & 0.200\\2 & 60.0 & 1/4 & 0.100\\3 & 90.0 &1/8 & 0.050\\4 & 120.0 & 1/16 & 0.025\\5& 150.0 & 1/32 & 0.012\\\end{array}](/tpl/images/0332/8503/2bb43.png)